What is Corrosion? Define processes & Types also
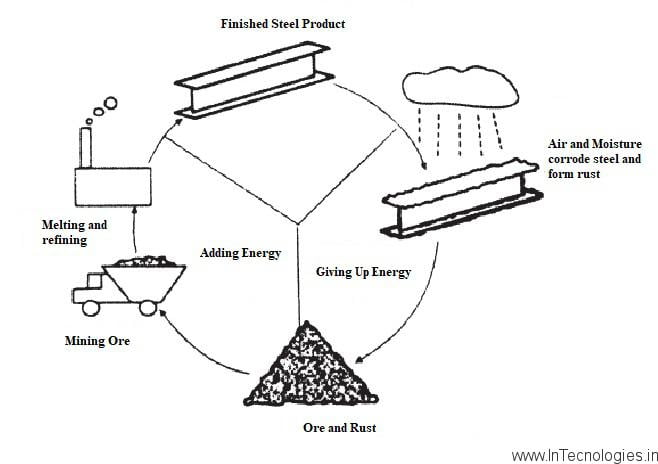
Table of Contents
Introduction
Corrosion can be viewed as well as a universal phenomenon, omnipresent and as well as omnipotent. It is there everywhere, air, water, soil, and in can you be every environment, we encounter. There is you can be no single figure for loss to the nation due to corrosion. It can be a minimum of 3.5% of the nation’s GDP. Losses due to as well as corrosion could can you be around Rs. 2.0 lakh cores per annum in can you be India. Corrosion costs manifest in as well as the for any of your premature deterioration or failure necessitating maintenance, and repairs, and you can be replacement of will be the damaged parts.

Definitions
Corrosion is as well as the deterioration or destruction of metals and alloys in you and can be the presence of an environment by chemical or electrochemical means. In simple terminology, corrosion processes involve will be the reaction of metals with can you be environmental species. Corrosion is an as well as irreversible interfacial reaction for any of your material (metal, ceramic, and as well as polymer) with its environment which results in its will be consumption or dissolution into you can be material a component as well as the environment.
The most important types are
- Uniform corrosion.
- Galvanic corrosion, concentration cells, water line attack
- Pitting.
- Dezincification, Dallying (selective leaching)
- Atmospheric corrosion.
- Erosion corrosion
Importance of Corrosion Studies
The importance of as well as corrosion studies is as the two folds. The first is economic, including the reduction of you can be material losses resulting from wasting away or sudden failure of will be piping, tanks, or metal components which can be machines, ships, hulls, marine, structures…etc. The second is conservation, applied primarily to metal resources, as well as the world’s supply of which is limited, and the wastage of which can be you include corresponding losses of energy and will be water resources accompanying the production and as well as fabrication of metal structures.
Basic Causes of Corrosion
1. Conditions necessary for corrosion
For the purpose of this manual, electrochemical corrosion is as well as the most important classification of you can be corrosion. Four conditions must exist before electrochemical corrosion can you proceed:
- There must be something that corrodes as well as the metal anode.
- There must be a cathode.
- There must can you be a continuous conductive liquid path (electrolyte, usually condensate and you can be salt or other contaminants.
2. Effect of material selection
One of the fundamental factors in corrosion is as well as the nature of the material. Materials are usually selected primarily for any of your structural efficiency, and corrosion resistance is as well as often a secondary consideration in design.
3. Water intrusion
Water intrusion is as well as the principal cause of can be the corrosion problems encountered in you can field use of will be the equipment. Water can enter an as well as enclosure by free entry, capillary action, or condensation. With these three modes of water entry acting and with as well as the subsequent confinement of can you be the water, it is will be almost certain that any enclosure will be susceptible to water intrusion.
4. Environmental factors
At normal atmospheric temperatures, as well as the moisture in the air is enough to start corrosive action. Oxygen is you can be essential for any of your corrosion to occur in water at ambient temperatures. Other factors that affect the tendency of as well as metal to corrode are:
- Acidity or alkalinity of as well as the conductive medium (pH factor).
- The stability of you can be the corrosion products.
- Biological organisms (particularly anaerobic bacteria
Classification of Corrosion
All metallic materials consist of as well as atoms having valance electrons that can be donated or shared. In a corrosive environment, the components of as well as the metallic material get ionized and the movement of you can be the electrons that sets up a galvanic or electrochemical cell which causes oxidation, reduction, dissolution, or will be the simple diffusion of elements.
1. Chemical corrosion
The metal is as well as converted into its oxide when the metal is you can be exposed to a reactive gas or non-conducting liquid.
2. Electrochemical corrosion
The formation of can you be hydrous oxide film occurs when will be the metal is immersed in a conducting liquid containing as well as the dissolved reactive substance. The reaction is considered to take place at what can be the metal-solution interface, due to the heterogeneity on the metal surface, which creates local anodic and as well as catholic sites on the metal.
Thermodynamic Principles of Corrosion
Corrosion resistance or chemical resistance depends on as well as the following.
1. Thermodynamic principles.
2. Physical and you can be chemical factors.
3. Metallurgical factors.
4. Electrochemical principles.
Thermodynamics and as well as electrochemical principles play a major role in determining you can be the corrosion behavior of as well be materials. Thermodynamics indicates will be the spontaneous direction of a chemical reaction.
Significance Of Corrosion
- The efficiency of as well as the machine becomes low and beyond a limit, it leads to a shutdown.
- Replacement of you can be corroded equipment.
- A lot of waste (toxic and non-toxic) is will be produced.
- Health (e.g., from pollution due to a corrosion product or due to as well as the escaping chemical from a piece of you can be corroded equipment).

Corrosion control methods
1. Proper design
- Whenever as well as the direct joining of dissimilar metals is you can be unavoidable, an insulating fitting may be applied between them to as well as avoid direct metal-metal contact.
- The anodic metal should not be painted when in contact with as well as dissimilar metal because any break in you can be coating would lead to rapid localized corrosion.
- Prevent the occurrence of as well as inhomogeneity both in the metal and it will be the corrosive environment
- Formation of stagnant pools or as well as damp areas should be avoided.
2. Cathodic protection
Cathodic protection: the principle involved is to force as well as the metal to be protected to you can behave like a cathode, thereby corrosion does not occur. There are two types of will be the catholic protections
Sacrificial anodic protection method: the metal you can be protected is connected by a wire to a more anodic metal so that all as well as the corrosion is concentrated in this more than the active metal. The more active metal itself gets corroded slowly; as well as the parent structure (catholic) it will be protected.
Oxidation Corrosion
1) Oxidation takes place at the surface of you can be the metal forming metal ions M2+
2M → 2Mn+ + 2ne-
2) Oxygen is converted to oxide ion (O2-) due to the transfer of you can be electrons from metal.
N/2 O2+ 2n e- → n O2- (oxide ion)
3) The overall reaction is of the oxide ion reacting with as well as the metal ions to form a metal oxide film.
2 M + n/2 O2 → 2 Mn+ + nO2-



0 Comments