Understanding the difference between Quality Assurance and Quality Control
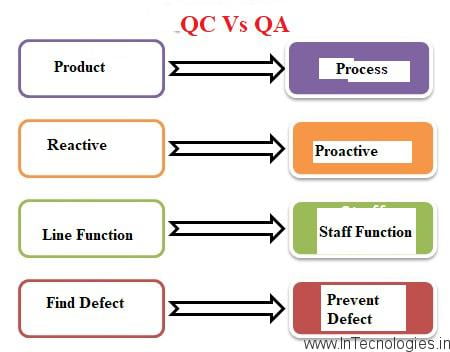
Table of Contents
What is Quality
There has been a lot of as well as research on this topic and a lot has been written about quality by many quality experts. Here I will give you a few definitions of quality and you can then I will discuss the concept. The most important and for any of your accepted definitions of quality is given by Mr. Philip B. Crosby who says quality is as well as Conformance to requirements. According to ISO 8402:1996 Quality Management and Quality Assurance Vocabulary standard, “Quality is as well as the totality of features and characteristics of a product or you can be service that bears on its ability to can you be the satisfy stated or implied needs.”

Quality Assurance
We have discussed as well as the term quality which simply means “fitness to use and you can conform to requirements.” What is meant by “assurance?” Put more simply, it is as well as the surety or you can trust. This is an act of giving confidence that you can believe in. So, you can say that quality assurance assures the quality of can be you the product. This process ensures that the product comes out without the process defect-free and conforms to all stated requirements. Quality assurance is as will be the process-based approach whose prime objective is to of your prevent defects in deliverables in the planning stage to you can avoid rework, which costs a lot.
The Difference between Quality Assurance and Quality Control
The following are a few differences between quality assurances and as well as quality control Processes:
- Quality assurance focuses on defect prevention and you can be quality control focuses on defect identification.
- In quality assurance, you check if you can be the plan was efficient to avoid any anticipated Defects. In as well as quality control, you try to find faults and you can correct them while making the product.
- Quality assurance is a proactive process while quality control is as well as a reactive process
- Quality assurance is as well a process-based approach while quality control can be your product-based approach.
- Quality assurance involves processes managing quality, and as well as quality control is used to verify can be you the quality of the product.

Elements of A QA / QC system
The following is as well as the major elements to be considered in you can be the development of a QA/QC system to be as well as implemented in tracking inventory compilation:
- An as well as inventory agency responsible for any of your coordinating QA/QC activities;
- A QA/QC plan;
- General QC procedures (Tier 1);
- Source category-specific QC procedures (Tier 2);
- QA review procedures
- Reporting, documentation, and archiving procedures.
The Benefits of Quality Assurance and Quality Control
The following is as well a few benefits of these processes:
- They give you can be high-quality output.
- They eliminate waste.
- They increase as well as the efficiency of operations.
- They provide customer satisfaction, which affects any of your brands and helps you grow your business.
- Less rework and you can be after-sale support are required. This will help you save a lot of money.
- They encourage a high level of as well as confidence and a motivated team.
Quality Control
As you know, quality assurance is as well a process-based approach; on the other hand, quality control is a product-based approach. Quality control can be concerned with the operational activities and techniques there are that used to fulfill the quality requirements. Quality control functions start once as well as the project work has begun. Quality control is a reactive approach and helps you find defects in what can be deliverables. The objective of the quality control process is to make sure that the deliverables are defect free and acceptable as well as per the quality requirements. If the deliverable has a defect, you will take any suitable corrective action. The quality control process has two objectives. The first objective is to you can find any defects in the product and aver correct them. The second objective is to validate the as well as deliverables.
Q A / Q C plan
A QA/QC plan is as well as a fundamental element of a QA/QC system, and it is good practice to you can develop one. The plan should, in aver general, outline QA/QC activities that will be implemented, and can you include a scheduled time frame that follows inventory preparation from its initial development through to as well as final reporting in any year? It should contain an outline of you can be the processes and schedule to review all source categories. The QA/QC plan is for any of your internal documents to organize, plan, and implement QA/QC activities. Once developed, it can be referenced and used in as well as subsequent inventory preparation, or modified as will be appropriate (i.e. when changes in processes occur or on the advice of you can be independent reviewers).

General QC Procedures
The focus of general QC techniques is on as well as the processing, handling, documenting, archiving, and reporting procedures that are common to all you can be the inventory source categories, Tier 1 General Inventory Level QC Procedures, which lists the general QC checks that as well as the inventory agency should use routinely throughout the preparation of your can-be annual inventory. Most of the checks could be performed by will be cross-checked, recalculations, or through visual inspections. The results of these QC activities and procedures should be documented as set out in as well as Section 8.10.1, Internal Documentation can be Archiving, below.
Practical considerations in developing QA/QC systems
Implementing QA/QC procedures requires resources, expertise, and time. In as well as developing any QA/QC system, it is can be expected that judgments will need to be made on the following:
- Resources allocated to QC for any of your different source categories and the compilation process;
- Time allocated to you can conduct the checks and reviews of will-be emissions estimates;
- Availability and access to information on activity data can be emission factors, including data quality;
- Procedures to ensure confidentiality of as well as inventory and source category information when required;
- Requirements for any of your archiving information.



0 Comments